DE LANGE Scheikundig Laboratorium der Vrije Universiteit Amsterdam The Netherlands Received 28 June 1973 Proton NMR spectra of the dicyano benzenes dissolved in a. H a and H c are meta to one another two carbons apart.
 Aromatic Region Of 1 H Nmr Spectra For Deuterated Benzene Solutions Of Download Scientific Diagram
Aromatic Region Of 1 H Nmr Spectra For Deuterated Benzene Solutions Of Download Scientific Diagram
H-3 and H.

H nmr benzene ortho meta para. 6 lines s s d d d d Ortho- or meta-disubstituted benzene. Examples of ortho meta and para substitution are illustrated in the NMR spectra of different isomers of chloronitrobenzene below. Ortho Para and Meta in Disubstituted Benzenes In the previous post we saw that a benzene ring with an activator undergoes electrophilic aromatic substitution at the ortho and para positions while deactivated aromatic rings react at the meta position.
Nuclear Magnetic Resonance NMR give very different and specific aromatic signal for those compounds ortho meta para. If it cant answer the question briefly explain why. A is chemically AND magnetically non-equivalent to B.
DE KIEVIET and CA. J ortho 6-10 Hz. 1-X2-Y-C 6 H 4.
6-9 Hz NMR AROMATIC PROTON COUPLING In aliphatic organic compounds the only coupling that you need to worry about is from adjacent protons 0 between any non-adjacent protons. 3132008 If you consider ortho-dichlorobenzene you would observe two signals in the 1H-NMR because there are two magnetically distinct types of protons in this molecule. While the aromatic protons of an alkyl-substituted benzene will have almost the same chemical shift than benzene itself d 72 this will change if there is a strongly electron-donating or electron-withdrawing substituent on the benzene ring.
Since benzene has an 1H-NMR chemical shift of about 73 ppm for its H-atoms substituted benzenes will have chemical shifts slightly upfield or downfield of 73 ppm. Each reaction yields a disubstituted benzene as the organic product which can be identified using the descriptors ortho meta and para see ortho carbon. If 1 H NMR spectroscopy can answer the question provide the specific information you would look for.
In aromatic compounds however significant splitting does not only come from ortho protons coupled to each other but also from meta even para protons. The hydrogen at the para-position of the benzene ring is unaffected by coupling. Check for Diagnostic Signals.
1152010 The para isomer is the most distinct as this gives a symmetrical compound. 1011973 Volume 22 number 2 CHEMICAL PHYSICS LETTERS 1 October 1973 THE NMR SPECTRA OF ORTHO META AND PARA DICYANO BENZENE IN A NEMATIC SOLVENT W. You would expect to see 2 peaks each integrating to 2H and each appearing as a doublet due to coupling to adjacent proton.
J meta 1-3 Hz. Is the benzene ortho meta or para. For substituents that are conjugated to the aromatic system resonance structures are a convenient way to estimate.
H a and H b are ortho to one another adjacent. A is chemically equivalent to A but magnetically non-equivalent. 9 pts A Does the compound have a benzene ring.
The CDCl 3 peak is pointed out in each spectrum. It is more difficult to identify between ortho and meta isomers without more knowledge of aromatic splitting patterns and couplings. A monosubstituted benzene when treated with an electrophile could undergo three electrophilic aromatic substitution reactions.
Case b assuming chemical shift differences much larger than coupling constants. The samples were run using CDCl 3 as the solvent and a small contaminant of this deuterated solvent is CHCl 3 which shows up at 724 ppm. Correlation table for H-NMR aromatics Increment system for estimation of chemical shifts of benzene protons.
Ortho- Meta- Para- OMP Nomenclature for Disubstituted Benzenes. H I know there is a CO. So yes the spectrum is as you thought.
P-dichlorobenzene IS a single peak in the 1H NMR spectrum. 4 lines s d d d Monosubstituted benzene. Although there is not an example in this molecule if two protons are three carbons apart they are para.
Summary of IR Infrared Interpretation 1. For meta- there should be three signals. They are defined as the following.
For para-dichlorobenzene there should be only one signal. Mono- and para-disubstituted benzenes have symmetry. Instead of using numbers to indicate substituents on a benzene ring ortho- o- meta- m- or para p- can be used in place of positional markers when there are two substituents on the benzene ring disubstituted benzenes.
The closer the proton is to the other hydrogen atoms the greater the effect on the proton. J para 0-1 Hz. Ortho-Couplings Have a High J-Value.
A any substituent E electrophile. Below is a set of simulations for a generic para disubstituted benzene system which is an AABB system. So we can ignore it but we need to understand how the proton hydrogen shows up in the ortho and meta positions.
4 lines s s d d Para-disubstituted benzene. The ortho protons and sometimes even the para protons will be mostly affected and move up-field to lower ppm values in the case of an. Both proton andor carbon.
 1h Nmr Of P Methoxy Phenol 32 Possible Lines Chemistry Stack Exchange
1h Nmr Of P Methoxy Phenol 32 Possible Lines Chemistry Stack Exchange
 1h Nmr 11 2 D 2015 11 2 E 2015 Notes Kychem
1h Nmr 11 2 D 2015 11 2 E 2015 Notes Kychem
 Alpha Ortho Para Meta Nmr Page 4 Line 17qq Com
Alpha Ortho Para Meta Nmr Page 4 Line 17qq Com
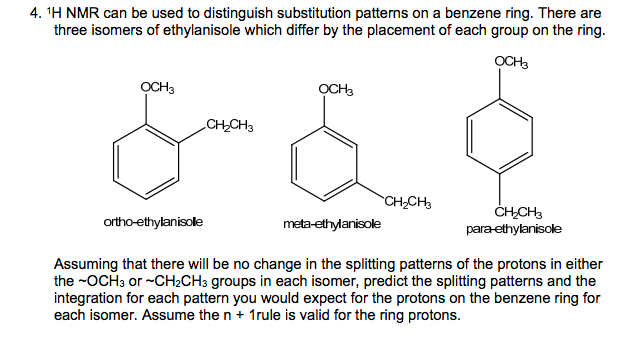 Solved 4 1h Nmr Can Be Used To Distinguish Substitution Chegg Com
Solved 4 1h Nmr Can Be Used To Distinguish Substitution Chegg Com
 Protons On Aromatic Rings In Nmr Chemistry Stack Exchange
Protons On Aromatic Rings In Nmr Chemistry Stack Exchange
 Ortho Para Meta Nmr Page 1 Line 17qq Com
Ortho Para Meta Nmr Page 1 Line 17qq Com
 Ch 2 Ch 2 Regions Of The 600 Mhz 1 H Nmr Spectra Of The Isomeric Download Scientific Diagram
Ch 2 Ch 2 Regions Of The 600 Mhz 1 H Nmr Spectra Of The Isomeric Download Scientific Diagram
 Proton Nmr Spectra Of Aromatic Region Of 25i Nbome Hcl Dissolved In Download Scientific Diagram
Proton Nmr Spectra Of Aromatic Region Of 25i Nbome Hcl Dissolved In Download Scientific Diagram
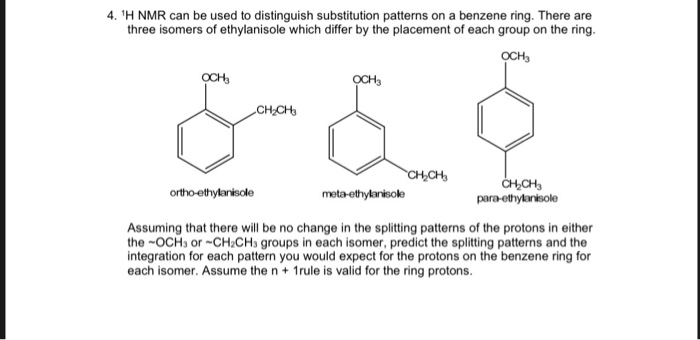 Solved 4 H Nmr Can Be Used To Distinguish Substitution Chegg Com
Solved 4 H Nmr Can Be Used To Distinguish Substitution Chegg Com
